Extensive vs intensive property – In the realm of chemistry and physics, the concepts of extensive and intensive properties play a crucial role in understanding the behavior of matter. Extensive properties, such as mass and volume, depend on the amount of matter present, while intensive properties, such as density and temperature, remain constant regardless of the amount.
This guide will delve into the fascinating world of extensive and intensive properties, exploring their definitions, characteristics, applications, and the intriguing relationships between them.
Extensive and Intensive Properties: Extensive Vs Intensive Property
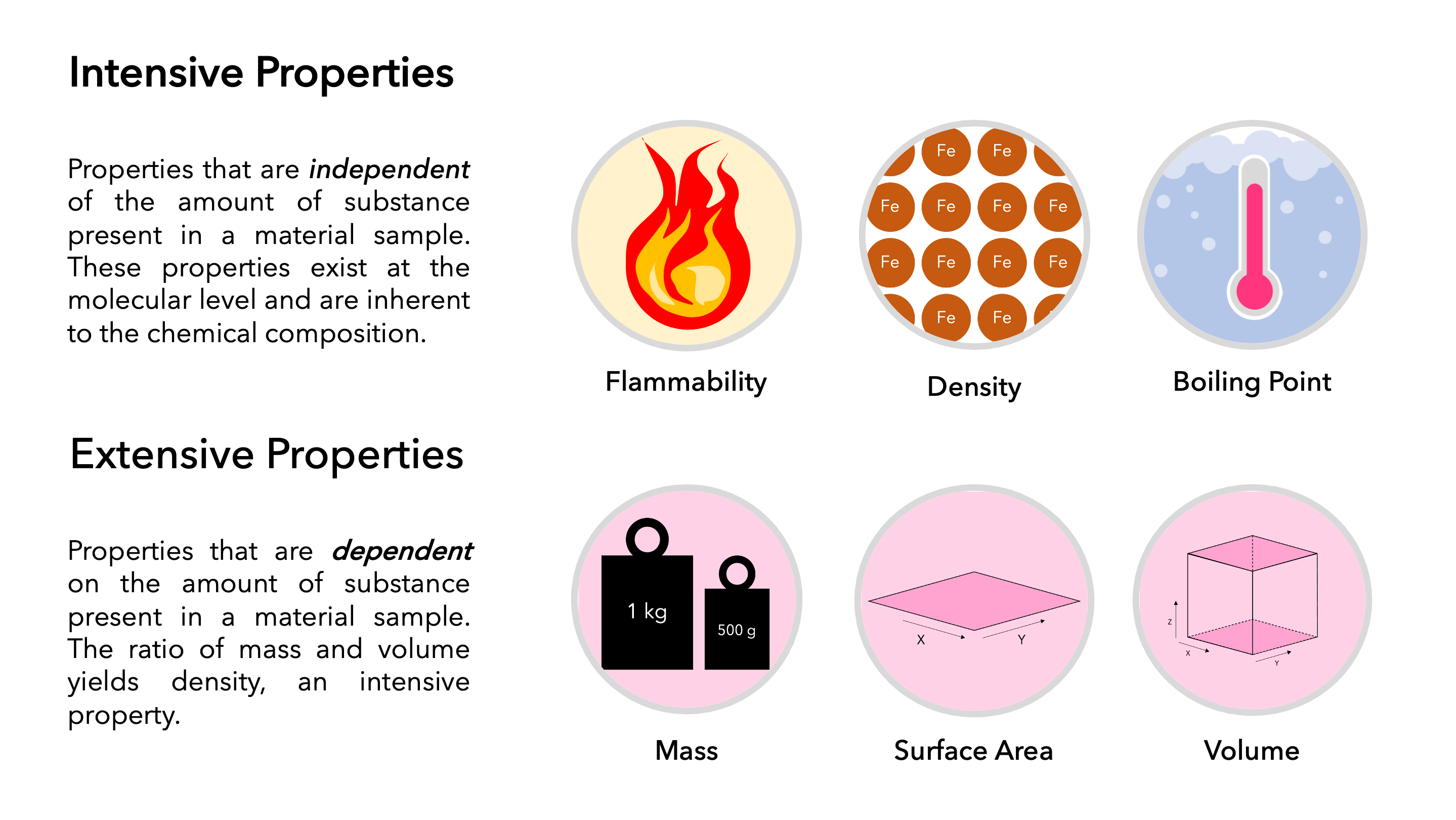
In chemistry, properties of matter can be classified as either extensive or intensive. Extensive properties depend on the amount of matter present, while intensive properties do not.
Definitions
Extensive property:A property that depends on the amount of matter present. Examples include mass, volume, and energy.
Intensive property:A property that does not depend on the amount of matter present. Examples include temperature, density, and solubility.
Characteristics
Extensive properties are additive, meaning that the total property of a system is the sum of the properties of its components. Intensive properties, on the other hand, are not additive. The total intensive property of a system is not necessarily equal to the sum of the intensive properties of its components.
Extensive properties are also proportional to the amount of matter present. This means that if the amount of matter in a system is doubled, the extensive properties of the system will also double. Intensive properties, on the other hand, are not proportional to the amount of matter present.
This means that if the amount of matter in a system is doubled, the intensive properties of the system will not necessarily double.
Applications
Extensive properties are used in a variety of real-world applications. For example, mass is used to determine the weight of objects, volume is used to determine the capacity of containers, and energy is used to power machines.
Intensive properties are also used in a variety of real-world applications. For example, temperature is used to measure the hotness or coldness of objects, density is used to determine the mass of objects per unit volume, and solubility is used to determine the amount of a substance that can be dissolved in a solvent.
Calculations, Extensive vs intensive property
Extensive properties can be calculated by adding the properties of the components of the system. For example, the mass of a system is the sum of the masses of its components. The volume of a system is the sum of the volumes of its components.
The energy of a system is the sum of the energies of its components.
Intensive properties cannot be calculated by adding the properties of the components of the system. Instead, intensive properties must be measured directly.
Relationships
Extensive and intensive properties are related to each other. For example, the density of a substance is the mass of the substance per unit volume. The density of a substance is an intensive property, but it is calculated using two extensive properties (mass and volume).
Extensive properties can be converted to intensive properties and vice versa. For example, the mass of a substance can be converted to its density by dividing the mass by the volume. The density of a substance can be converted to its mass by multiplying the density by the volume.
Examples
The following table lists some common extensive and intensive properties:
| Extensive Property | Intensive Property |
|---|---|
| Mass | Temperature |
| Volume | Density |
| Energy | Solubility |
Here are some additional examples of extensive and intensive properties:
- Extensive properties: length, area, height, number of atoms, number of molecules
- Intensive properties: boiling point, melting point, refractive index, viscosity, electrical conductivity
Closing Notes
As we conclude our exploration of extensive and intensive properties, it becomes evident that these concepts provide a fundamental framework for understanding the behavior of matter. By comprehending the distinctions and connections between these properties, scientists and engineers can gain valuable insights into the workings of our universe.
Expert Answers
What is the difference between extensive and intensive properties?
Extensive properties depend on the amount of matter present, while intensive properties do not.
Extensive and intensive properties are two important concepts in chemistry. Extensive properties depend on the amount of matter in a sample, while intensive properties do not. For example, mass and volume are extensive properties, while temperature and pressure are intensive properties.
When it comes to finding the best mortgage rates, it’s important to consider both extensive and intensive properties. The amount of money you borrow is an extensive property, while the interest rate is an intensive property. By considering both of these factors, you can find the best mortgage rate for your needs.
Best mortgage rates Rochester NY can help you find the best mortgage rate for your needs.
Can extensive properties be converted to intensive properties?
Yes, extensive properties can be converted to intensive properties by dividing by the amount of matter.
What are some examples of extensive properties?
Mass, volume, energy, and heat capacity are examples of extensive properties.
What are some examples of intensive properties?
Density, temperature, pressure, and solubility are examples of intensive properties.